What can stop cheatgrass from spreading? Not much. In the 1940s, Aldo Leopold warned this invasive plant posed a grave threat to western US habitats. Since then, it has spread. And spread. Today, it infests 50 million acres of sagebrush steppe habitat.
That’s not only bad for wildlife like sage grouse, mule deer and pygmy rabbits that rely on intact sagebrush steppe for survival, it’s bad for ranchers who are battling this invasive weed and for anyone living in areas where cheatgrass-fueled fires rage. Areas that once experienced fire an average of once every 30-150 years may experience fires an average of once every 3-5 years once cheatgrass moves in.
Cheatgrass creates a vicious fire cycle. Cheatgrass roots grow when it is still cool outside, earlier in the spring than most native plants in sagebrush habitat, and they continue growing later into the fall. The cheatgrass plant produces an extensive root system that is able to take up more water and nutrients before native plants have even started to grow. Cheatgrass then dries out by late spring or early summer and provides fuel for wildfires that clear established native plants nearby, making room for more cheatgrass to seed. Each year, the process restarts. Native plants, unable to compete and unable to withstand frequent fire, soon give way to cheatgrass monocultures.
“Big sagebrush is killed by fire,” says Chuck Warner of The Nature Conservancy (TNC) in Washington. “The only way to reestablish it is by seed. Then it takes about ten years to mature and be reasonable habitat for sage grouse or pygmy rabbit. Fire intervals of 5-7 years don’t give it much of a chance.”
Traditionally, herbicide has been the primary method of removing cheatgrass and, though some herbicides can be effective against cheatgrass, they only take out the grown plants, leaving seeds in the soil that will grow and need to be sprayed the following year.

“The herbicide doesn’t really get rid of the cheatgrass, whereas the bacteria will,” says Ann Kennedy of the USDA-Agricultural Research Service.
That’s why research by Kennedy, supported by The Nature Conservancy in Washington, is aimed at attacking the root of the problem — using soil microbes that inhibit the growth of the plant’s root system, allowing native plants to compete and diminish the number of cheatgrass seeds in the soil over time without impacting native plants or crops.
The Solution Right Under Our Noses
The world of soil microbes is a hidden realm all around us that gives scientists additional options to study. Advances in this realm are changing everything from our understanding of the human body to the way that we create materials, and they are set to change conservation as well.
“There is a genetic potential in the soil that we have not even begun to explore,” Kennedy explains. “What this research shows is that we can actually go into the soil and find those organisms or isolates that have the traits we want and use that trait selectively.”

She has been researching microbes for more than 30 years and one day, when she saw a stunted wheat plant in a field, she decided to find out what was going on in the soil nearby. She and her colleagues isolated 25,000 microscopic organisms native to the soil of Washington State and tested their effects on crops and non-native plants, eventually isolating a promising bacterium known as D7 for further testing as a biocontrol.
Testing Microbes in the Field
Working in various locations in Washington and Oregon, including the Nature Conservancy’s Moses Coulee preserve, Kennedy field tested D7 to see how it would perform in varied microclimates and what were the best conditions to apply the bacteria. When it comes to application, timing is everything.
“We have this cold loving organism that we have to be careful that we don’t put it on when the predators from the microbial community would eat it up,” says Kennedy. “It’s like the food web even at the microbial level. There are some paramecium and protozoa that would just as soon eat the microbial community as look at it.”
Kennedy’s team found that mid-October is the start of the best time to apply the bacteria, however, conditions must be right — air temperatures can be no higher than 50o F and rain needs to be coming soon in the forecast.

The bacteria need rain to drive them into the soil where it can establish; if there aren’t sufficient rains in October and November to drive the bacteria into the soil, freezing weather makes it difficult to apply the bacteria, but Kennedy has found a surprising solution to problems with frozen equipment.
“Once you get to December, the spray nozzles tend to freeze and they can crack and you’ll be out in the field and all the material goes right through that spray nozzle that is cracked so you can’t get it on. Or, if you’re going to apply by airplane, you can’t get the airplane to fly because the weather is so poor,” Kennedy explains. “I use those hand warmers that you can crack. We give those to people and you can actually warm up your nozzles on the spray rig with that.”
D7 and some other promising microbial candidates that Kennedy is working with inhibited not only cheatgrass growth, but also the growth of another invasive, medusahead — without having a negative impact on native plants, wildlife, or crops.
“A big concern was that animals would eat it,” says Warner, “but we found that this biocontrol, when sprayed on surface of ground or plants is gone after 48 hours except for the parts that get into the soil. It’s very safe for wildlife.”

And it’s cost effective. Application of the bacteria costs an estimated $8-10 per acre and once you’ve applied it successfully, you don’t need to apply it again. The bacteria establish for the next few years, restricting the growth of cheatgrass and allowing the desired plants to take over. Application of herbicides to kill off cheatgrass costs an estimated $15 per acre every year, indefinitely.
“By year three we’re seeing a 50% reduction in the cheatgrass in almost every plot that we put the bacteria in,” Kennedy notes, “except for where the bacteria didn’t survive.”
What’s the Catch?
D7 doesn’t directly kill cheatgrass. It inhibits the growth of cheatgrass roots at a critical time in the plant’s development, giving native plants a chance to compete. This isn’t a bad thing per se, but it does require a change in expectations as compared to a traditional herbicide.
“A lot of times, when people put out an herbicide, in a week they can see the dying plant and then with the weed suppressive bacteria it really takes several years,” Kennedy observes. “The grandchildren [of the microbes] have to grow and they’re the ones that inhibit the weed. In the first year we see a little bit of inhibition, but not like if you were to spray an herbicide, it takes many years for the bacteria to work into the soil and get ahold of the roots and inhibit them.”
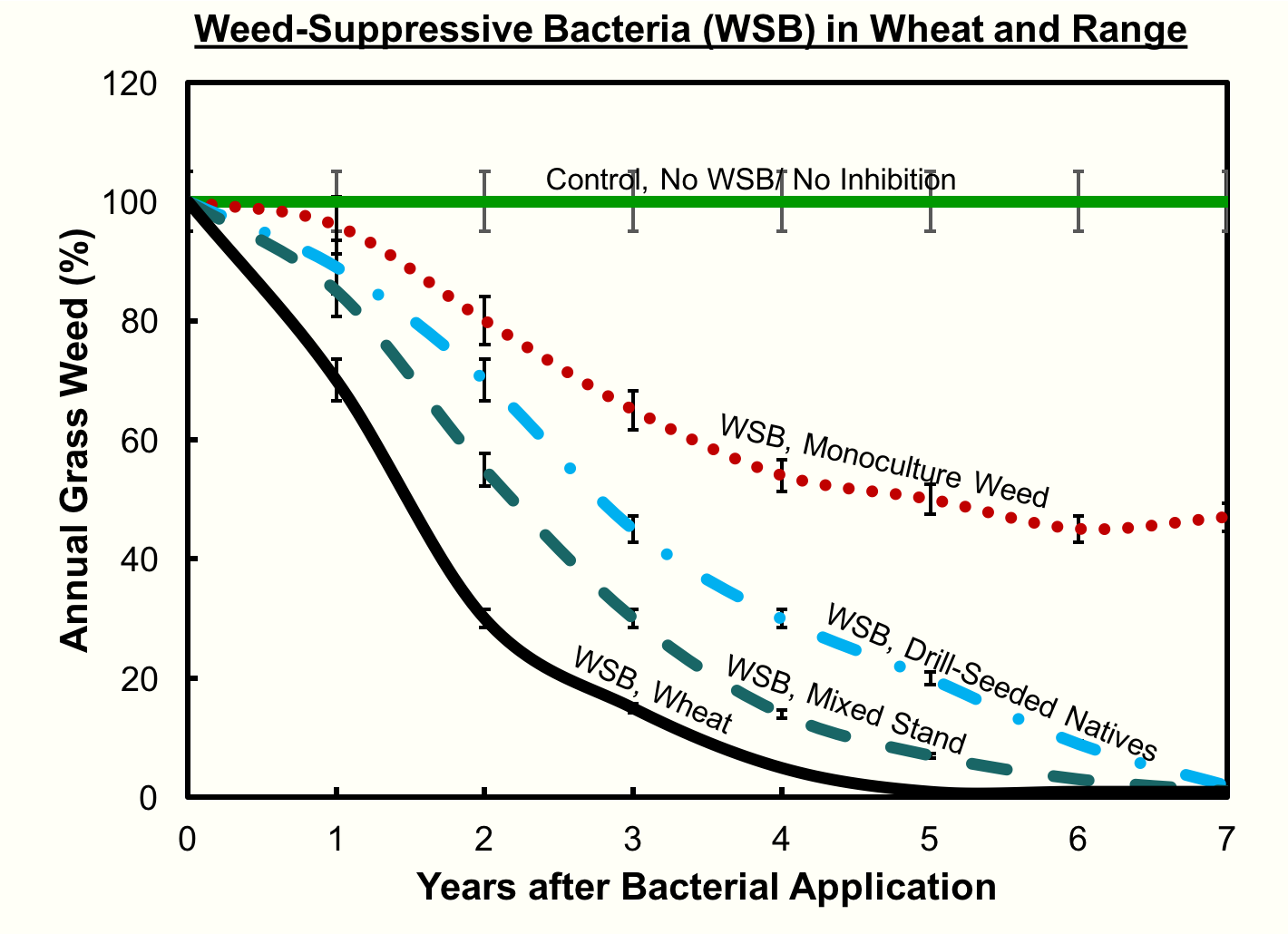
Furthermore, Kennedy’s field tests discovered that, where cheatgrass has been established as a monoculture for a long time, the void left by the removal of cheatgrass is often filled by broadleaf weeds. This is because there is no seed bank of native plants in the soil, but the broadleaf seeds remain. Extra work may be required to reestablish the seed bank of native plants, for instance by using a traditional herbicide on the broadleaves and planting native seeds.
“If you can add a desirable plant whether wheat or natives into the system and have it grow well,” says Kennedy, “the bacteria has a much better chance of reducing the cheatgrass.”
However, it’s also important that these introduced microbes not last in the soil indefinitely. Although they are native to part of Washington, if they remained, they might cause unforeseen problems.
For instance, a bacterium known as 110 was introduced to soybeans in the 1950s to help plants fix nitrogen. It is now known that there are bacteria that could do the job better, but the 110 bacterium has been so successful in outcompeting other bacteria that it remains in the soil to fix nitrogen for soybeans all over the world to this day.

“I swore I would never put anything into the soil that would survive a long time,” says Kennedy, “An added bacterium would have to come in and do its thing for 4-5 years and then die off. We’ve selected these bacteria to be benign in the soil, so they don’t fight, they don’t have the antibiotics to fight against somebody who might come and want to eat them.”
Sowing the Seeds of Restoration
The next step is to spread the use of these “bacterial herbicides.” D7 has already been approved by the EPA and another bacterium is under review. They will eventually be made commercially available for land managers and owners who are looking for a long-term solution to the spread of cheatgrass and medusahead.
With more than 50 million acres of cheatgrass to combat, it is unlikely that the invasive will be eradicated any time soon, but these biocontrols will be important tools for beating back the invasion, protecting and restoring areas of sagebrush steppe, and could be used to create buffer zones to contain wildfires.
Kennedy will publish the data from her cheatgrass control studies with TNC within the year and continues to look at other collections and isolates of bacteria that could work against other invasive plants.
“It’s not just a one-time deal,” Kennedy explains, “we’re actually showing that the concept works quite nicely. We can find other grass weed inhibitory bacteria in the same situation where we found the other ones [i.e. where there is stunted wheat plant].”




Thanks for the information! I only have 10 acres but I think I have a lot of cheatgrass that I would love to get rid of. How do I get the D7 and how do I apply it?
is D-7 on the market ?
We recently bought 6 acres outside of Cody WY and have 6 acres of cheatgrass! Do you know if it would work in a dryer climate if put down as prescribed in the article?
Hi Tina, I recommend contacting ARS (Agricultural Research Service) to find out if they have tested in a drier environment and to find out what products contain the biocontrol agent. Thank you!
Is there an update on the use of bacterial herbicides for getting rid of cheat grass?
Hi Debra, Ann Kennedy retired. ARS is continuing the research, but hasn’t yet published any new results. Thank you for your interest!
Hi Lisa. Great article on Cheatgrass. I’m writing a report on the invasion of Cheatgrass in the Mammoth Lakes area. Research indicates that cheatgrass burns 10 times hotter than any native species. Is there any research that indicates that a temperature that hot would have a direct affect on the D7 bacteria? All the Best! George
Hi George, Thank you for the question! I don’t know of any studies on that question and Ann Kennedy has retired. You might try contacting one of the researchers from this Webinar to see if you can learn more: http://greatbasinfirescience.org/events-webinars/2017/8/28/using-weed-suppressive-bacteria-to-control-invasive-annuals
Thank you to the writer, researchers, Ann Kennedy and Dixie Dringham for tackling this cheatgrass problem and spreading the word. I am so grateful that you are aware of and working on this. As I travel around this beautiful country it amazes me to know about the ongoing efforts to keep it natural and wonderful and true, wherever possible.
Thanks for your work in this area. I was wondering if you have explored using microbes to reduce weed seed banks. We have a real problem with oxeye daisy and Queen Ann’s lace and other weeds in the Willamette Valley where it crowds out our natives and some rare plants. We can kill the weeds but with the seed bank being so large it takes many applications of herbicides to begin to reduce the seed bank. Using microbes to reduce the weed seed bank would go a long way to reducing the use of herbicides and the length of time to reestablish our natives. I would look to use native microbes that are generalist seed killers like you used for cheat grass. I am not worried if we kill off the native seed bank in the process because we can always go in and reseed with natives.
Thank you for the question Greg! Ann Kennedy has retired, but there are plans to continue her work at ARS, if the tests continue to go well, I expect to see more experiments using microbes to control a wide variety of plants. I have a story coming soon about researchers in Oregon who are working on improving sagebrush restoration success using a variety of seeding technologies – including seed coatings that can include beneficial microbes. Here is an earlier paper on their work (mostly behind a paywall, but the abstract is free) that you might be interested in: http://onlinelibrary.wiley.com/doi/10.1111/rec.12332/abstract
I have several acres of campground in the Reno NV area that had no cheat grass 4 years ago and is now fully infested. Would you be interested in trying D7 in this environment? How can I get access to the D7 since the article suggests it’s not year commercially available?
Hi Cindy, Thank you for your interest – I suggest you get in touch with the Northwest Sustainable Agroecosystems Research Unit (the part of the ARS that is working on this research) to find out if they could test in your area or if there is a way for you to obtain D7. Their info is here: https://www.ars.usda.gov/pacific-west-area/pullman-wa/northwest-sustainable-agroecosystems-research/
I’m not sure I would support the direction of using microbial herbacides to combat invasion of cheatgrass. Today they state it as a promising solution. Yet time and again the so-called “solution” ends up posing as unforeseen s that are every bit as challenging or Bigger and the microbial method is stated to take years and then may only assist, not radicate the cheatgrass.
Would it not be better to burn the soil and then replant or use a Preen type that abates seed germination?
Maybe it would be better to instead burn the soil, replant and see if this method seems faster
Hi Alison, Thank you for your comment! As I understand it from talking to Dr. Kennedy and others, fire benefits cheatgrass, since cheatgrass grows back faster than the native species after a fire. It is true that when not carefully planned, some “solutions” have turned out for the worse. I take comfort in knowing that Dr. Kennedy is very careful in her planning and testing to make sure that this microbial control measure will not damage the native ecosystem.
I think when people think about Science or conservation they’re not thinking of native grasses and weeds and the important part they play in grasslands and prairies, the importance of them as fire blocks and to prevent soil erosion. Good work Lisa, Kennedy, et al. thank you!
Where can property owners obtain the D7 herbicide for private use? We own 34 acres that border the Crooked River National Grasslands and have large swaths of cheatgrass we need to remove. I had planned to use a traditional herbicide, but would rather try the D7.
Thank you Ann Kennedy. I truly hope this works as advertised and creates no possible future problems with who knows what.
Interesting, interesting article! Would like to have Ms Kennedy do some projects for us in Eastern Oregon!
I live in Redmond Oregon . Cheat grass is z big problem and appears to be growing in between the sage brush and trees. What can i do to eradicate this weed from growing ?
Hi Mary, The biocontrol is not commercially available yet. I suggest contacting Oregon Invasive Species Council’s grass mapper for advice: http://www.oregoninvasivespeciescouncil.org/additional-resources/
where can one find out more information? on D7 or the study?
Hi Tracey, Thank you for your interest! The results of the study haven’t been published yet. I’d recommend watching Ann Kennedy’s publications page for updates or checking with ARS later this year to see if the study has been published – https://www.ars.usda.gov/people-locations/person?person-id=2989