Using magnets, beakers, and, well, packing tape, TNC NatureNet Science Fellow Robert Higgins, working in Eric Schelter’s lab at the University of Pennsylvania, describes an environmentally friendly approach to extract the rare-earth minerals that are critical to most modern technologies, including cell phones and computers, as well as the wind turbines and electric vehicles that are the hope of a green energy future.
The Gist
Higgins’ and his colleagues’ work, published in the journal Angewandte Chemie International Edition, describes a novel process using magnetic fields and temperature differences to make rare earth extraction and separation not only more efficient and cost-effective, but also substantially less toxic and polluting.
Specifically, these researchers were able to efficiently — and selectively — separate heavy rare earths like dysprosium from lighter rare earths such as lanthanum. The key discovery was that combining a magnetic field with a decrease in temperature caused the heavy rare earth elements to selectively crystallize first.
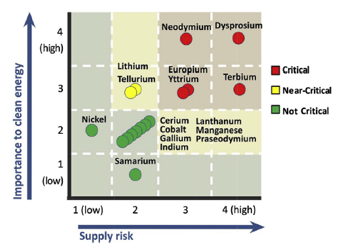
The most striking and efficient separation researchers documented was taking a 50/50 mixture of lanthanum and dysprosium and getting back 99.7% dysprosium in one step—a “100 percent boost” compared to the same low-temperature method but without using a magnet.
This is especially significant because dysprosium is the most important rare earth for green technology, but is also the one most at risk for its supply to run out.
The Big Picture
One of the key challenges in working with rare earths is that current processes for mining and separating the elements into useable amounts are expensive, inefficient, energy-intensive, and can be highly toxic and polluting.
The 17 elements of the Periodic Table, known as the rare earths, are not necessarily hard to find and are fairly abundant in mountainous areas around the world, especially in China, Russia and the United States. But because they are not found in concentrated deposits like gold or silver, separating and extracting them from each other and surrounding ore is an inefficient process that generates a huge footprint and a large amount of acidic waste.
In some ways, rare earths represent a terrible irony at the heart of so-called sustainable and green technologies. Wind turbines, low-energy light bulbs, electric and hydrogen-powered cars — they all currently require rare earth elements that can only be extracted using industrial processes that are anything but sustainable or environmentally friendly.
“The work Dr. Higgins is doing as a NatureNet Science Fellow is really important to reduce the environmental waste related to current rare-earth metal extraction,” says Martha Rogers, a natural capital economist with TNC’s Center for Sustainability Science, “but it also critical to the future of the clean energy industry. Together, we’re currently working on ways to measure the cost-effectiveness of this new separation process with the hopes of not only motivating its adoption in current operations but also in future operations.”
The Takeaway
“The faster we can find new ways of performing separations more effectively,” notes Higgins, “the faster we can improve some of the geopolitical, environmental and climate issues that are associated with rare earth mining and recycling. This is a really important starting point towards a cleaner and more circular rare earth metals economy.”
References:
Higgins, R., Cheisson, T., Cole, B., Manor, B., Carroll, P., and Schelter, E. (2019), Magnetic Field Directed Rare‐Earth Separations. Angew. Chem. Int. Ed.. Accepted Author Manuscript. doi:10.1002/anie.201911606
Binnemans, K., et al. (2013), Recycling of rare earths: a critical review. Journal of Cleaner Production. Volume 51, 2013, Pages 1-22. https://doi.org/10.1016/j.jclepro.2012.12.037


Join the Discussion